Last Updated on August 18, 2021 by OJ Maño
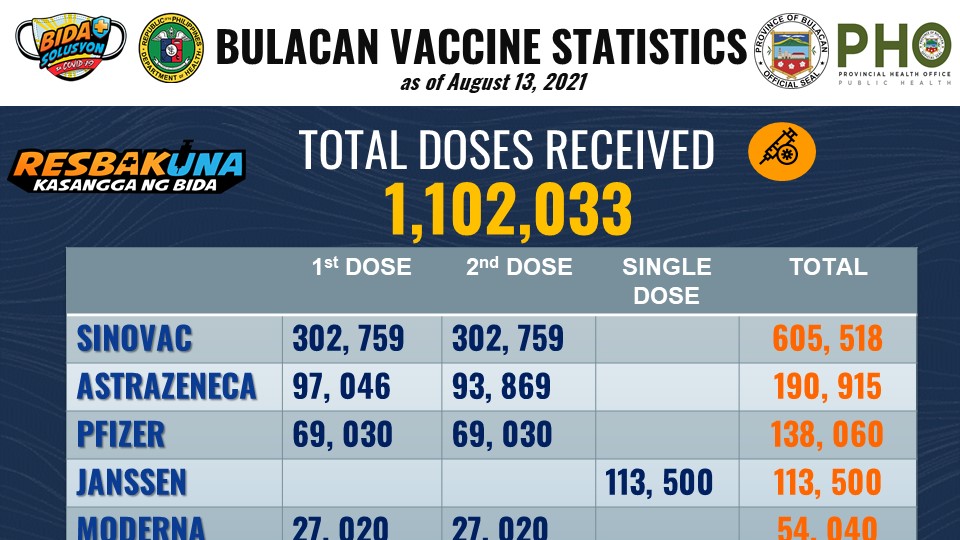
As of August 15, five (5) brands of COVID-19 vaccines are being used to innoculate Bulacan residents and front-liners. These are Sinovac, Pfizer, AstraZeneca, Moderna, and Janssen. According to National Task Force against COVID-19 Chief Implementer and Vaccine Czar Secretary Carlito G. Galvez Jr. (NTF Vaccine Czar), more brands and supplies are coming.
All brands of COVID-19 vaccines available in Bulacan have Emergency Use Authorization (EUA) approvals by the Philippine Food and Drug Administration (FDA). A EUA is an authorization issued for unregistered drugs and vaccines in a public health emergency. By Executive Order No. 121 of the President of the Philippines, the FDA Director-General authorizes the issuance of the EUA.
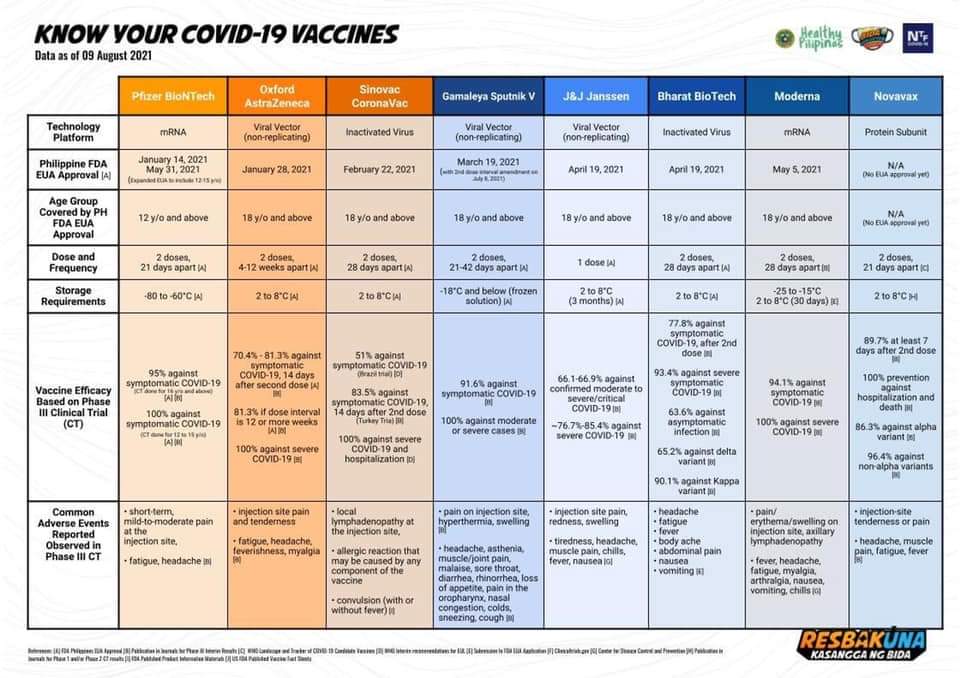
The Philippine FDA authorizes 8 COVID-19 vaccines. These are Pfizer-BioNTech, AstraZeneca, Coronavac, Sputnik V, Janssen, Covaxin, Moderna, and Sinopharm.
COVID-19 Vaccines Available in Bulacan
Pfizer-BioNTech COVID-19 Vaccine (BNT162b2)
The Pfizer–BioNTech COVID-19 vaccine is used to provide protection against COVID-19, caused by infection with the SARS-CoV-2 virus. The vaccine is used to reduce morbidity and mortality from COVID-19. This is given in two doses with an interval of three to four weeks between doses. The vaccine is given by intramuscular injection.
Pfizer–BioNTech is an mRNA-based COVID-19 vaccine developed by the German biotechnology company BioNTech and Pfizer, an American drug company. Distribution and storage are logistical challenges because the vaccine needs to be stored at extremely low temperatures or minus 70 degrees Celsius.
AstraZeneca COVID-19 Vaccine (ChAdOx1-S)
The Oxford–AstraZeneca COVID-19 vaccine is a viral vector vaccine for the prevention of COVID-19. Oxford University and AstraZeneca developed it. The vaccine is given by intramuscular injection and comes in two doses. The World Health Organization (WHO) recommends 8 to 12 weeks between doses for optimal efficacy.
Sinovac COVID-19 Vaccine
CoronaVac, also known as the Sinovac COVID-19 vaccine, is an inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech. The vaccine is given by intramuscular injection and given in two doses. The World Health Organization (WHO) recommends an interval of 3 to 4 weeks between doses.
As of July 2021, CoronaVac was the most widely used COVID-19 vaccine globally, with 943 million doses delivered.
In the Philippines, CoronaVac’s Sinovac is the top-most available vaccine. The Chinese government donated around one million doses of Sinovac last March 2021. The Philippine government, LGUs, and the private sector have procured around 25 million more doses of the same brand.
Johnson & Johnson’s Janssen COVID-19 Vaccine (Ad26.COV2-S (recombinant))
The Janssen COVID-19 vaccine or Johnson & Johnson COVID-19 vaccine is used to protecting against infection by the SARS-CoV-2 virus. The vaccine is given by intramuscular injection. This is the first-ever single-dose anti-COVID vaccine in the world. The vaccine can remain viable for months in a standard refrigerator.
COVID-19 mRNA Vaccine (nucleoside modified) [COVID-19 Vaccine Moderna]
The Moderna is an mRNA-based COVID-19 vaccine used to protect against infection by the SARS-CoV-2 virus. The vaccine is given by intramuscular injection. It consists of two doses. The World Health Organization (WHO) recommends an interval of 28 days between doses.
Moderna requires standard medical refrigerator storage of 2–8 °C (36–46 °F) for up to thirty days or minus 20 °C (−4 °F) for up to four months.
Other Possible COVID-19 Vaccines To Be Available in Bulacan Soon
Sputnik V Gam-COVID-Vac COVID-19 Vaccine
Sputnik V or Gam-COVID-Vac is an adenovirus viral vector vaccine for COVID-19 developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia. The vaccine is given by intramuscular injection. It consists of two doses with an interval of 21 days between doses.
What to do if you develop side effects after getting vaccinated?
Possible side effects are pain, redness, and swelling in the arm where you got the shot. You may also experience tiredness, headache, muscle pain, chills, fever, and nausea. These side effects happen within a day or two of getting the vaccine. They are normal signs that your body is building protection and should go away within a few days.
According to World Health Organization (WHO), you may take paracetamol or other painkillers if you develop side effects such as pain, fever, headache, or muscle aches after vaccination.
The Centers for Disease Control and Prevention (CDC) in the US does not recommend, however, that people take such over-the-counter medications or antihistamines to prevent side effects before receiving the coronavirus vaccine.
“You can take these medications to relieve post-vaccination side effects if you have no other medical reasons that prevent you from taking these medications normally,” the CDC states. “It is not recommended you take these medicines before vaccination for the purpose of trying to prevent side effects.”
Sources
- Know your Vaccines (vaccine Matrix: Current Evidence): Department of health website. Know Your Vaccines (Vaccine Matrix: Current Evidence) | Department of Health website. (n.d.). https://doh.gov.ph/vaccines/know-your-vaccines.
- Food and Drug Administration. (2021, August 13). List of FDA issued Emergency Use Authorization – Food and Drug Administration. https://www.fda.gov.ph/list-of-fda-issued-emergency-use-authorization/.
- NBC Chicago. (2021, May 22). Can you take IBUPROFEN after the COVID Vaccine? NBC Chicago. https://www.nbcchicago.com/news/local/can-you-take-ibuprofen-after-the-covid-vaccine/2516487/.
- World Health Organization. (n.d.). COVID-19 vaccines advice. World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice.
- Centers for Disease Control and Prevention. (n.d.). Pfizer-BioNTech COVID-19 Vaccine overview and safety. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html.
- Centers for Disease Control and Prevention. (n.d.). Johnson & JOHNSON’S Janssen Covid-19 Vaccine overview and safety. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html.
- SINOVAC: Supply Vaccines to Eliminate Human Diseases. (2021, August 10). Http://Www.Sinovac.Com/Index.Php?Lang=en. http://www.sinovac.com/index.php?lang=en
- Information about the Moderna COVID-19 Vaccine. (2021, June 11). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html